38 draw a ph scale and label water hydrochloric acid and sodium hydroxide
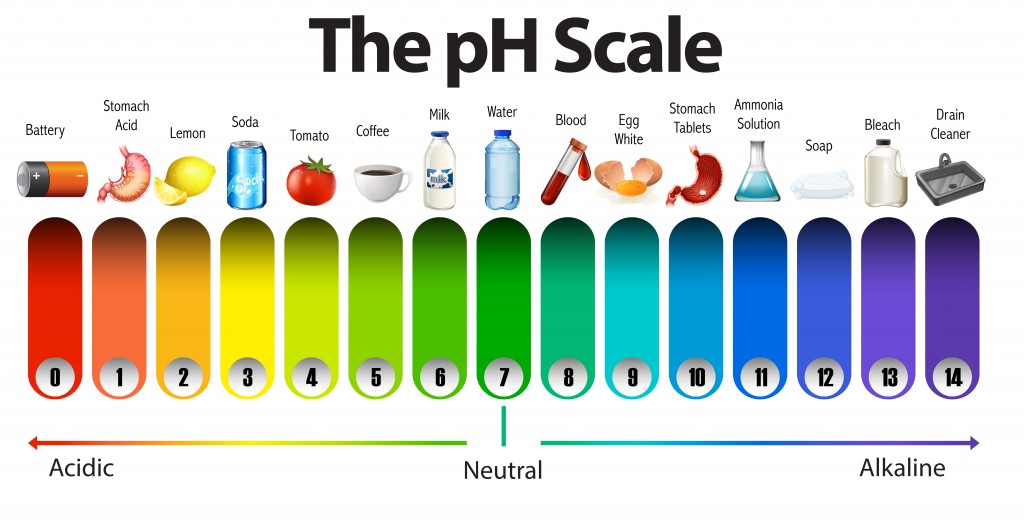
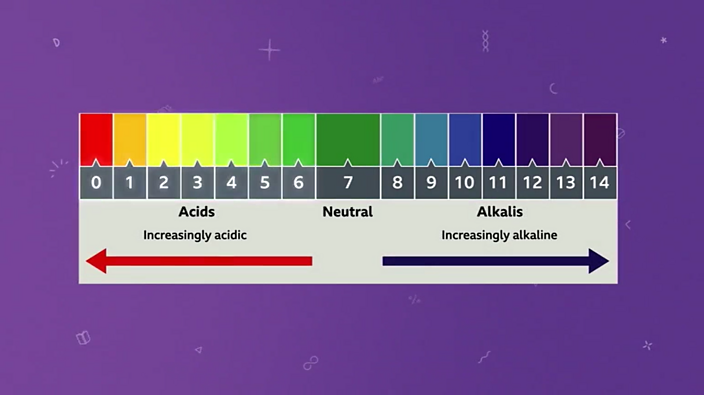
pH Scale | U.S. Geological Survey Acid Rain and the pH Scale The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Draw a pH scale and label water hydrochloric acid and sodium hydroxide ... See attached scan for answer which is pdf file Scan 0009 or 0.01M HCl is pH of 2, water is pH of 7 and NaOH concentrated is pH of 14. So standard water is neutral, HCl at this concentration is quite acidic and concentrated NaOH is very alkaline. Download pdf pdf pdf Advertisement
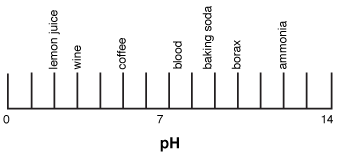
pH Of Samples Experiment - CBSE Class 10 Science Practicals - BYJUS What is pH? pH is a measure of hydrogen ion concentration to determine the alkalinity or acidity of a solution. If the pH value of a solution is less than 7 it is an acidic solution. If the pH value of a solution is greater than 7 it is a basic solution. If the pH value of a solution is equal to 7 it is a neutral solution.
Draw a ph scale and label water hydrochloric acid and sodium hydroxide
lab 4 Flashcards | Quizlet Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. Verified answer. Other Quizlet sets. Educational Strategies in an Inclusive Classroom. 22 terms. Timothyeng416. Psych 310 Chapter 8. 34 terms. samanthakcourville. draw and label the ph scale - minecraftskullonfirepainting Draw a pH scale and label water hydrochloric acid and sodium hydroxide in. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base. PH 0 2. Label each situation as an acid or a base. The pH inside cells 68 and the pH in the blood 74 are both very close to neutral. draw and label the ph scale - myra-biol The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is. Label each situation as an acid or a base. Draw a pH scale and label water hydrochloric acid and sodium hydroxide in their general areas on the scale - 727803. The pH inside cells 68 and the pH in the blood 74 are both very close to neutral.
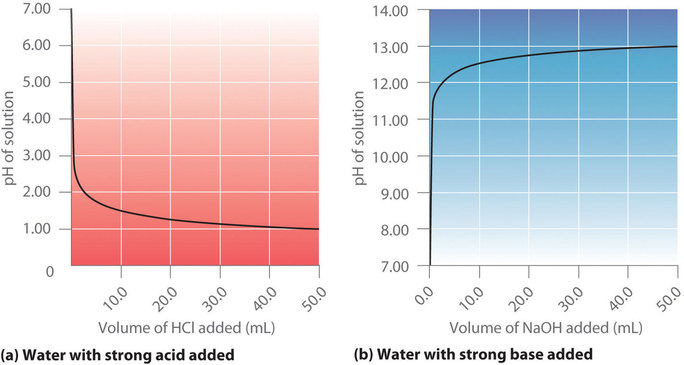
Draw a ph scale and label water hydrochloric acid and sodium hydroxide. Practical activity - carrying out a titration - BBC Bitesize To carry out an accurate titration using dilute hydrochloric acid, dilute sodium hydroxide solution, and phenolphthalein indicator. Titration is a practical technique used to determine the amount ... pH curves (titration curves) - chemguide The graph is showing two end points - one at a pH of 8.3 (little more than a point of inflexion), and a second at about pH 3.7. The reaction is obviously happening in two distinct parts. In the first part, complete at A in the diagram, the sodium carbonate is reacting with the acid to produce sodium hydrogencarbonate: 17.4: Titrations and pH Curves - Chemistry LibreTexts (a) When 0.20 M HCl is added to 50.0 mL of distilled water, the pH rapidly decreases until it reaches a minimum at the pH of 0.20 M HCl. (b) Conversely, when 0.20 M NaOH is added to 50.0 mL of distilled water, the pH rapidly increases until it reaches a maximum at the pH of 0.20 M NaOH. (CC BY-SA-NC; Anonymous by request) 13.3: Finding the pH of weak Acids, Bases, and Salts For a strong acid such as hydrochloric, its total dissociation means that [HCl] = 0, so the mass balance relationship in Equation 13.3.3 reduces to the trivial expression Ca = [Cl-]. Any acid for which [HA] > 0 is by definition a weak acid. Similarly, for a base B we can write. (13.3.4) C b = [ B] + [ H B +] Note.
Titration of phosphoric acid with sodium hydroxide 0.1M solution of phosphoric acid titrated with 0.1M solution of strong base. pK a1 =2.15, pK a2 =7.20, pK a3 =12.35. Titration curve calculated with BATE - pH calculator. It is interesting to mention, that phosphoric acid can be titrated as triprotic - if PO 43- anion is precipitated first using metal ions (for example Ca 2+ or Ag + ): After ... NaOH and HCl Titration Curves | Selecting Indicators So pH of the initial aqueous HCl solution is 1. If you are not familiar with calculating pH of HCl, refer this tutorial. Initial HCl amount of 25 cm 3 is 0.0025 mol. To neutralize the all HCl amount, same amount of NaOH is required because HCl and NaOH reacts according to the 1:1 ratio. science hw.pdf - Liz Griffith 1. Describe one way in which water helps ... 2. Relate the structure of water to its ability to act as a solvent. Water can dissolve solutes in solutions and is the Earth's greatest solvent. 3. Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. 4. Compare and contrast solutions and suspensions. Give examples of each. Solutions, such Chapter 6 vocab and assessments Flashcards | Quizlet Ionic bond electrical attraction between two oppositely charged atoms or groups of atoms Van der Waals force attractive forces between molecules 1. Sodium has 11 protons and 11 neutrons in its nucleus. Draw a sodium atom. Be sure to label the particles ... 2. Explain why carbon monoxide (CO) is or is not an atom
pH Scale | U.S. Geological Survey The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic. Biology Chapter 6 Glencoe Science Flashcards | Quizlet Ionic bond electrical attraction between two oppositely charged atoms or groups of atoms Van der Waals force attractive forces between molecules 1. Sodium has 11 protons and 11 neutrons in its nucleus. Draw a sodium atom. Be sure to label the particles ... 2. Explain why carbon monoxide (CO) is or is not an atom pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens. What is the measure of the size of a body or region in three ... - BRAINLY What is the measure of the size of a body or region in three-dimensional space
How to Find the Concentration When You're Given the pH pH = − log [H3O+] The pH of a solution is equal to the negative logarithm of the hydronium ion (H3O+) concentration. Example 1: Find pH from [H3O+]. In a 1.0 L sample of 0.1 M hydrochloric acid (HCl) the concentration of hydronium ions is 1 × 10 -1.
Draw a pH scale and label water, hydrochloric acid, and sodi - Quizlet Find step-by-step Biology solutions and your answer to the following textbook question: Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale..
Rocky Shore Zones Diagram | Quizlet Mid-Tide Zone. This zone is covered and exposed for equal time during the tide cycle. High Tide Zone. This zone is mostly exposed - it is only submerged at the point of high tide and for a longer period of time during extremely high tides. Splash Zone. This area is nearly always exposed. It only will be submerged at very high tides. This is rare.
14.9: The pH and pOH Scales - Chemistry LibreTexts p H is usually (but not always) between 0 and 14. Knowing the dependence of p H on [ H 3 O +], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the p H scale. The pH scale is the range of value s from 0 to 14 that describes the ...
The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly.
The "reaction of sodium hydroxide and hydrochloric acid" Despite the aggression of the sodium hydroxide and hydrochloric acid, the reaction was a wonderful one. And its equation reads as follows: HCl + NaOH = NaCl + H₂O + Q Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
Titration of a Strong Acid With A Strong Base - Chemistry LibreTexts At the equivalence point, equal amounts of H + and OH - ions will combine to form H 2 O, resulting in a pH of 7.0 (neutral). The pH at the equivalence point for this titration will always be 7.0, note that this is true only for titrations of strong acid with strong base.
3. Draw a pH scale and label water, hydrochloric acid, and sodium ... Explanation: pH sacle may be defined as the scale that determines the pH of acids and bases. 0 represent the neutral compounds. The compounds that pH less than 7 acts as acid whereas the compounds that have pH greater than 7 acts as base. Here, water is neutral, Hydrochloric acid (HCl) is acidic and sodium hydroxide (NaOH) acts as base.
The pH scale - BBC Bitesize The pH scale measures how strongly acidic or alkaline a solution is using a set of values from pH 0 to pH 14. When pure water is dropped into a solution of universal indicator, the indicator stays...
Acid-Base Pairs, Strength of Acids and Bases, and pH - Purdue University Strong and Weak Acids and Bases . Many hardware stores sell "muriatic acid" a 6 M solution of hydrochloric acid HCl(aq) to clean bricks and concrete. Grocery stores sell vinegar, which is a 1 M solution of acetic acid: CH 3 CO 2 H. Although both substances are acids, you wouldn't use muriatic acid in salad dressing, and vinegar is ineffective in cleaning bricks or concrete.
draw and label the ph scale - myra-biol The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is. Label each situation as an acid or a base. Draw a pH scale and label water hydrochloric acid and sodium hydroxide in their general areas on the scale - 727803. The pH inside cells 68 and the pH in the blood 74 are both very close to neutral.
draw and label the ph scale - minecraftskullonfirepainting Draw a pH scale and label water hydrochloric acid and sodium hydroxide in. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base. PH 0 2. Label each situation as an acid or a base. The pH inside cells 68 and the pH in the blood 74 are both very close to neutral.
lab 4 Flashcards | Quizlet Draw a pH scale and label water, hydrochloric acid, and sodium hydroxide in their general areas on the scale. Verified answer. Other Quizlet sets. Educational Strategies in an Inclusive Classroom. 22 terms. Timothyeng416. Psych 310 Chapter 8. 34 terms. samanthakcourville.













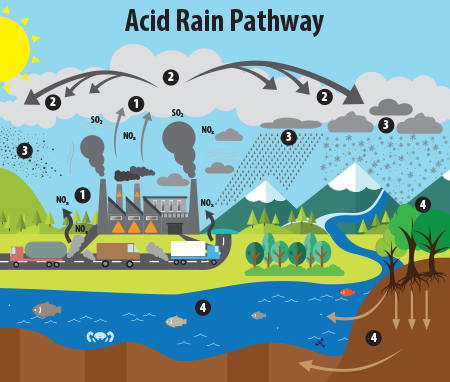
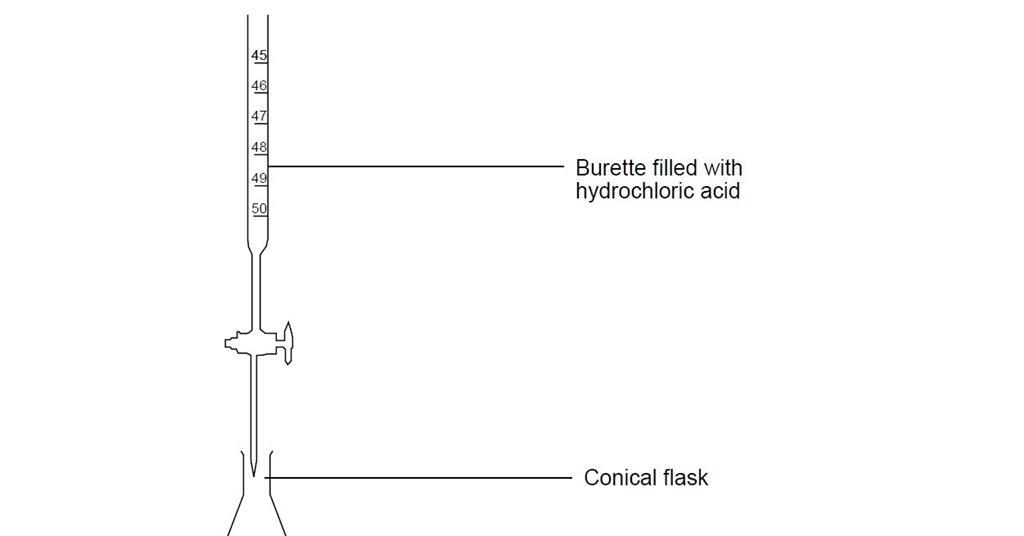

:max_bytes(150000):strip_icc()/definition-of-ph-in-chemistry-604605_final-5c8fac8446e0fb00017700d1.png)

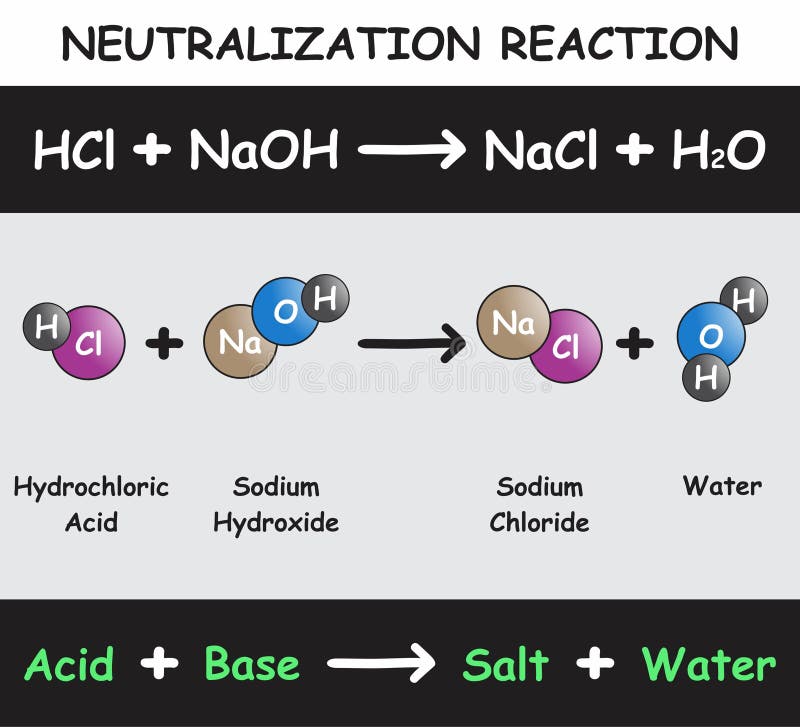



Post a Comment for "38 draw a ph scale and label water hydrochloric acid and sodium hydroxide"